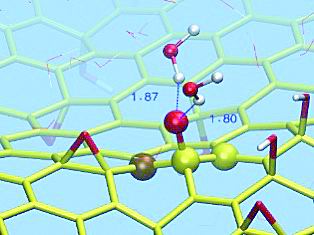
The epoxy on the surface of graphene oxide migrates dynamically with the participation of water molecules. Yellow is a graphene carbocycle, red is an oxygen atom, and white is a hydrogen atom. Photo courtesy of Tu Yusong
Recently, Professor Tu Yusong's research group of the School of Physical Science and Technology of Yangzhou University and Shi Guosheng, a researcher of the School of Environmental and Chemical Engineering of Shanghai University, published a paper in the "Chinese Physics Express" to carry out theoretically combined experimental research on functional groups on the surface of graphene oxide The distribution law and its reasons were answered.
They found that when water molecules are adsorbed, graphene oxide can be transformed into an adaptive dynamic covalent material. Water molecules act as mediators and can reduce the energy barrier for oxygen migration in graphene oxide to a level similar to or lower than the hydrogen bonding energy of liquid water. The epoxy and hydroxyl functional groups can spontaneously break or reorganize the carbon-oxygen bond under the medium of water molecules to realize the dynamic migration of oxygen.
Epoxy "supported on water and stepped on stilts"
Tu Yusong introduced that graphene is a single layer of carbon atoms. Except for the application of a small amount of pure graphene, there are more graphene oxides in practical applications. There are two functional groups epoxy and hydroxyl groups directly above and below the single-layer graphene oxide, and some carboxyl functional groups are distributed on the edge of the graphene oxide .
"Since these oxidized functional groups are hydrophilic and graphene itself is hydrophobic, the staggered distribution of these two types of materials is an important reason why graphene oxide can be applied in many ways." Shi Guosheng said.
Although this functional group distribution has been known for a long time, the previous point of view is that the distribution is completely random. There is no breakthrough in the principle, which makes the nature of graphene oxide difficult to ponder, and also confuses further research.
Based on the density functional theory, the research team calculated that in the absence of water, the energy required to break the carbon-oxygen bond in the epoxy and hydroxyl groups on the graphene oxide surface is 5~6 of the hydrogen bond energy of the liquid water molecule. Times. However, if water molecules are added to the environment, the energy barrier for breaking carbon-oxygen bonds can be reduced to a level comparable to the hydrogen bonding energy of water molecules.
This means that water can easily grab oxygen from carbon, allowing oxygen to move freely at room temperature.
Tu Yusong's team further confirmed through computational molecular dynamics simulation that the carbon-oxygen bond in the hydroxyl group was originally difficult to break, but if there is just an epoxy around, the hydroxyl group can transfer protons (hydrogen ions) to this epoxy.
Or the epoxy encounters a water molecule, and the oxygen in the water molecule can instantly connect three hydrogens, and then release one hydrogen to a carbon-oxygen bond of the epoxy. At this time, the epoxy becomes a hydroxyl group. When the latter spit the hydrogen ions back to the water molecule, the broken oxygen will connect a carbon atom in the adjacent area to re-form the epoxy.
In this way, the two carbon-oxygen bonds of epoxy are like a pair of stilts, which can move continuously with the help of hydroxyl or water molecules.
"In previous studies in this area, most of the oxidized groups were regarded as isolated, and the hydroxyl group or epoxy group was studied individually." Tu Yusong told the China Science Journal, "And we regard epoxy, hydroxyl group and water molecule as one. Overall, the process of dynamic changes with mutual assistance." In computational molecular dynamics simulations, at normal temperature and pressure, the oxidized group can move spontaneously.
"This gives us a lot of confidence." Tu Yusong said.
Challenges in experimental testing
The research team thought of many ways to observe related phenomena through experiments and follow-up tests. Eventually, the in-situ infrared spectroscopy device of the Shanghai synchrotron radiation light source gave them the opportunity to realize the in-situ detection of graphene structures.
"The sensitivity of synchronous infrared detection is high, and there is no need to vacuum, you can do it." Shi Guosheng said.
In order to test what changes the graphene oxide group has when adsorbed by water molecules compared to a dry environment, they need to put the sample in a relatively dry environment and add water little by little. To this end, they designed a relatively narrow box to put the sample, using nitrogen blowing to keep the box evenly dry. At this time, the distribution of oxidized groups on the graphene surface changes very little.
"With just a little water, the distribution of epoxy has a periodic oscillation change." Tu Yusong said, "This verifies our results."
But peer reviewers' questions followed one after another.
"Graphene is stacked together in your experiment. How do you prove that the change in epoxy occurs along a single layer rather than between layers?" "The mechanism you call causes graphene oxide Does it degrade?"
To answer this to Tu Yusong and others one by one, he believes that theory can prove that the interlayer movement of epoxy needs to span a longer distance, which means a higher energy barrier. In the presence of water, the interlayer spacing becomes larger and the energy barrier further increases. This shows that oxidized groups can only migrate along the plane of the layer, not between the layers.
The weighing and composition analysis of the graphene film before and after the experiment also proved that not only did the weight not lose, but also the proportion of carbon and oxygen in the graphene did not change, the surface of the graphene shifted only the position of the oxidized group, and No degradation occurs.
Let Graphene "Move"
Tu Yusong believes that the greatest significance of this work is to challenge the previous understanding-graphene oxide is not static, but dynamic. The oxidation sites are highly correlated, and the epoxy, hydroxyl, and water molecules work together to achieve large-area oxygen migration.
"A lot of phenomena that were not understandable before are suddenly bright. Water, as a common environmental variable, is also inspiring for future related research." Tu Yusong said.
Professor Rodney Ruoff, an expert in graphene oxide and the director of the Multidimensional Carbon Materials Center of the Ulsan Institute of Science and Technology in South Korea, contacted Tu Yusong and invited him to go to South Korea to discuss their model and carry out relevant research cooperation issues with this model. In the discussion, with many years of experience, Rouff suggested that Tu Yusong should try to study the movement behavior of oxygen on the surface of graphene oxide.
This time, it was found that water has a greater influence on the movement behavior of oxygen. Not only is it of theoretical significance, but this new understanding of the "dynamics" of graphene is also very important for applications.
Previously, researchers also tried to design and produce some dynamic response covalent materials and related systems, but it will involve a certain reversible reaction, which requires a large change in pressure, temperature or pH conditions, and has high requirements for the reaction process.
"The production of graphene oxide is simple, as long as it absorbs a little water, it can realize the large-area migration and change of oxygen on the surface of graphene under normal temperature and pressure, and it can be more easily applied in the future." Shi Guosheng said.
For example, since graphene oxide groups can dynamically adapt to biomolecules adsorbed in the environment, and will not disturb the structure and characteristics of biomolecules, if applied to two-dimensional biomolecule adsorption probes, it can make The detection accuracy of related sensors is higher.
"In the future, we want to try to cooperate with other research groups. Through some observation methods, such as the neutron reflection device, it has advantages in looking at the dynamic behavior of light elements. Adding some separately designed devices can directly see the atomic level on graphene. Dynamic changes." Tu Yusong said. (â– Chi Han, a trainee reporter of this newspaper)
Lemon furniture series:
The products ranges from residential furnitures to office furnitures, hotel furnitures,restaurant furnitures,outdoor furnitures etc.We supply full product chain from low end to high end,satisfy all kinds of requirements no matter for market hot sale or customized products.With more than 20years experience,we had served customers from all over the world. The supermarkets we worked with are from many Top 3 brand of their countries. The hospitalities we have served around theworld include: Marriott,Sheraton, Shangri-La, Crowne Plaza, Hyatt, Hilton, Wyndham, Kempinski.GRAND MLLENNIUM InterContinental,Club Med Ramada,Holiday nn, Double Tree, Best Western Hotels and Resorts,and more.
Outdoor Patio Furniture,Bedroom Furniture,American Signature Furniture,About Office Furniture
Lemon Building Material Co., Ltd. , https://www.lbmaluminumpergolas.com