Recently, Xiao Jianping, a researcher of the Special Zone Research Group of Theoretical Catalysis and Innovation of the State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, teamed up with Professor Hou Yang from Zhejiang University and Professor Wu Gang from the State University of New York at Buffalo in the United States. Progress has been made in formic acid research.
The chemical reactions that occur on the surface of the solid catalytic material follow the Sabatier principle, that is, when the catalyst is highly reactive, the reaction is affected by elementary steps such as desorption, diffusion, and coupling, and the activity is low; when the catalyst is less reactive When the reactants are difficult to adsorb and dissociate and activate, the catalytic activity is relatively low. Therefore, in order to achieve the optimal reactivity of the catalytic reaction, a moderate reactivity should be selected.
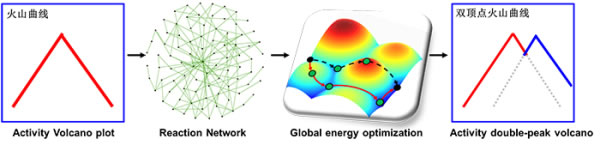
Studies have shown that the process of preparing formic acid by electrocatalytic reduction of carbon dioxide follows the reaction mechanism of formate (HCOO*), that is, carbon dioxide is protonated to obtain formate (HCOO*), and formate is protonated to obtain formic acid (HCOOH). In the past, researchers generally believed that the process of protonation of carbon dioxide to obtain carboxyl (COOH*) only produces CO, but not formic acid. However, this theoretical study shows that through the analysis of the reaction network and the energy optimization algorithm, a large number of metal surfaces at low voltage are used to obtain formic acid through the carboxyl (COOH*) process, which explains the special electrocatalytic reduction of carbon dioxide on metal Pd phenomenon. The theoretical research results are also consistent with the double volcano curve on the surface of basic lead carbonate (Nature Communications, 2020).
The study revealed that in the case of complex reaction networks, traditional volcanic curves are difficult to exist due to the difference in reaction paths. Instead, volcanic curves with multiple vertices coexist should be used instead. Relevant research results were published on Advanced Materials. The research work was supported by the National Natural Science Foundation of China, the Chinese Academy of Sciences Strategic Pilot Science and Technology Special (Class B) "Principles and Measurements of the Precise Construction of Functional Nanosystems" and the "Xingliao Talents Program" of Liaoning Province.
Kitchen Cabinets ,Kitchen Pantry Storage,Modular Kitchen Cabinets,Menards Kitchen Cabinets
Ningbo Oulin Import&Export Co.,Ltd. , https://www.oulinfurniture.com