
(Source: Princeton University official website)
As we all know, platinum is very expensive, and it is also one of the serious obstacles to the large-scale application of hydrogen fuel cells, one of the energy sources of electric vehicles, because hydrogen fuel cells require platinum. However, according to foreign media reports, the research team led by Bruce E. Koel, a professor in the Department of Biological and Chemical Engineering at Princeton University, has successfully found a cheaper alternative to platinum materials. The researchers said that they found a hafnium-based compound with a work efficiency of about 60% of the platinum material, but the cost is only about one-fifth of the platinum material.
The working principle of a fuel cell is to directly convert the energy stored in hydrogen atoms into electrical energy. NASA has long used fuel cells to power satellites and other space missions, and it is now also used in electric cars and buses. Hydrogen is the simplest and most abundant element in the planet and the universe.
At its most basic, a fuel cell generates electricity by splitting hydrogen into protons and electrons. Protons flow through the membrane and combine with oxygen to form water. The negatively charged electrons flow to the positively charged electrodes in the fuel cell. The flow of electrons is the current generated by the fuel cell and can provide power to the engine or other electronic equipment. And other materials as catalysts.
Catalysts are also used on hydrogen-fueled fuel cells to produce hydrogen. In the most ideal, fossil fuel-free case, renewable electrical energy can split water molecules (two hydrogen atoms and one oxygen atom) with the help of a catalyst, splitting water into oxygen and hydrogen, and the higher the efficiency of the catalyst, the decomposition The less energy the water needs.
Some advanced fuel cells, namely renewable fuel cells, combine the above two reactions. However, most fuel cells currently rely on hydrogen produced by independent systems to become hydrogen fuel cells. However, the best catalysts for the above two reactions are platinum group precious metals. Researchers believe that platinum is perfect. Platinum noble metals can catalyze chemical reactions quickly and efficiently to extract hydrogen, and these metals can withstand the harsh acidic conditions required for such reactions.
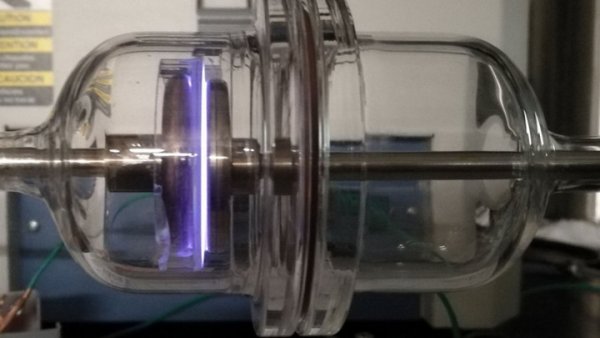
However, the problem is that platinum is scarce and expensive. However, researchers feel that hydrogen fuel cells used in electric vehicles and the like do not require platinum as a perfect material. Researchers have found that hafnium hydroxide is a good alternative, using nitrogen plasma (plasma is an ionized gas, a state of matter found in fluorescent lamps and the sun) that can inhale nitrogen atoms The material.
Previously, many materials were ignored because they were not conductive. However, the researchers found that the treatment of hafnium oxide with nitrogen plasma will form a thin film material, which can be used as a highly active catalyst and can survive under strong acid conditions. Although the efficiency of this hafnium-based film is only about two-thirds of that of platinum, the price is much cheaper. The researchers plan to test zirconium next, because zirconium is cheaper.
Although this material is very suitable for use in fuel cells, researchers believe that such materials are most valuable in systems that use catalysts to electrochemically decompose water to produce hydrogen as fuel. And the researchers emphasize that the discovery cannot commercialize new technologies at low prices in the near future. Currently, the process of making this material is very complicated and limited to the laboratory. Although they have determined the performance of such films, they still need to consider the engineering techniques required to mass-produce such materials. However, the study opened the door for further exploration of platinum alternative materials. (Author: Yuqiu Yun)
We supply various Lamp Holders And Lamp Bases, including B22 Lamp Base, E27 Light Holder, Lamp Holder E27 B22 2 in 1, Straight Lampholder, Angle Lampholder etc.
The below information will help you to know more about Lamp Holders And Lamp Bases.
Lamp holders, also known as light sockets, are fixtures used in lighting installations. They hold a bulb whilst also providing an electrical connection to it. It is essentially a connection between the wire and the bulb. Some lamp holders also provide support for a pendant or lampshade.
Which material is used for lamp holder?
The lamp holder is generally divided into plastic lamp holder, bakelite lamp holder, ceramic lamp holder and metal lamp holder etc.
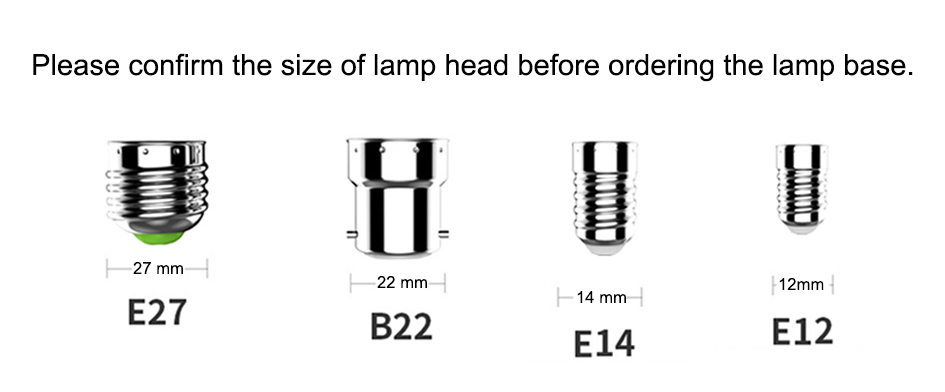
What is the difference between E14 and E27?
E14 is a 14 mm wide thin screw lamp holder. E27, on the other hand, is a 27 mm wide, thick screwed lamp holder. You can find out what type of bulb you need by measuring the lamp holder width.
There are many light bulb base sizes available.
C7 / E12 / Candelabra Base Bulbs ... typically used for chandelier-type fixtures and decorative lighting.
C9 / E17 / Intermediate Base Bulbs ... historically known as the old Christmas light bulb size.
Medium / E26 Bulbs (E27 in Europe) ... the same size as a standard household bulb.
If you want to know more about the products
in Lamp Holders And Lamp Bases, please click the product details to view
parameters, models, pictures, prices and other information about Lamp Holders
And Lamp Bases, Lamp Holders, Lamp Bases, Light Bulb Holder.
Whatever you are a group or individual, we will do
our best to provide you with accurate and comprehensive message about Lamp
Holders And Lamp Bases!
Lamp Holders And Lamp Bases ,Lamp Holders,Lamp Bases,Light Bulb Holder
NINGBO LONGFLIGHT INTELLIGENT TECHNOLOGY CO.,LTD , https://www.long-flight.com