 |
Figure 1: Photo of a newly developed all-solid Li-S battery. In order to make it easy to see the structure of the battery, a part of the negative electrode of metallic Li was intentionally peeled off.
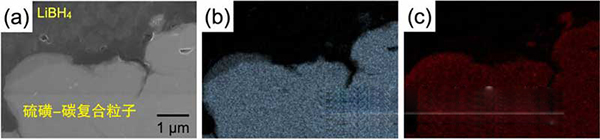
Figure 2: Field Emission Scanning Electron Microscopy Images of Cross Section of CS Composite Particles/LiBH4 Positive Layer (a), Distribution of S (b), Distribution of C (c). It can be seen that within the CS composite particles, C and S are in a mutually highly dispersed state. Moreover, CS composite particles and LiBH4 close together, forming a good contact interface.
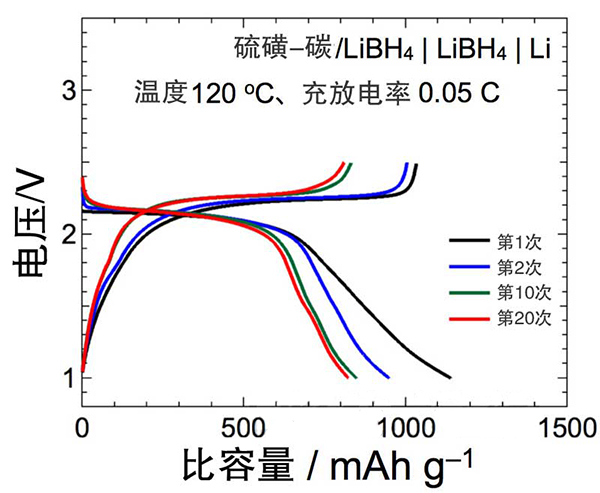
Figure 3: Charge and discharge curves of all-solid Li-S batteries. It was confirmed that the energy density per unit weight of the S positive electrode was maintained at 1590 Wh·kg-1 after 20 charge/discharge cycles (specific capacity of 820 mAhg−1), and remained at 1410 Wh·kg−1 after repeating 45 charge/discharge cycles ( With a capacity of 730mAhg-1), stable operation can be achieved.
Lecturer Yu Jiyi and lecturer of the Advanced Research Institute of Atomic and Molecular Materials, Tohoku University, Japan, research team of Furuo Shinichi. Through the joint research with the Institute of Metal Materials of Nihon University and Mitsubishi Gas Chemical Co., Ltd., he successfully developed the use of sulfur (S) positive electrode. All-solid Li-S battery with metal lithium (Li) anode (Figure 1). This was achieved using the technology developed by the research team and using complex lithium hydride borohydride (LiBH4) as a solid electrolyte.
The storage performance of a battery is determined by the combination of electrode materials. Compared with the electrodes used in the original battery, the S-anode and the Li-anode each have a theoretical capacity of 10 times or more, and it is possible to greatly improve the power storage performance. However, when an S-positive electrode is used as a battery with an organic electrolytic solution, discharge is likely to occur and the S-type positive electrode dissolves into the organic electrolytic solution. Therefore, after repeated charge and discharge, the storage performance is significantly reduced. To solve this problem, the world began to study solid electrolytes that can replace organic electrolytes. However, only a small part of the solid electrolyte can be equipped in the battery.
The research group of Tohoku University in Japan focused on the functionality of complexed hydrides as a solid electrolyte for batteries and developed a novel solid electrolyte based on complex hydrides. The complexed hydride LiBH4 exhibits lithium ion conductivity as high as 2×10 −3 S/cm at 120° C. Through this study, the research team successfully equipped the LiBH4 into the battery. It has been confirmed that after the all-solid-state Li-S battery was developed and repeatedly charged and discharged 45 times, the storage performance was not significantly reduced, and the unit weight energy density of the S cathode was more than 1,410 Wh·kg-1, which was comparable to that of the cathode materials used previously. It can work stably with 2 to 3 times more energy density.
Complex hydride solid electrolytes have many advantages over other inorganic solid electrolytes when used in batteries. The first is that lighter elements can be selected in terms of constituent elements, so that lightening can be achieved. Second, stability can be achieved over a large voltage range, so that a variety of electrodes can be used. The complexed hydride is easily deformed like a wax and can therefore be produced in an extremely simple manner by simply uniaxially pressing at room temperature. However, since sulfur is an insulator, in order to promote the reaction as a battery, it is necessary to form a good interface between sulfur and carbon (C) as a conductor and an electrolyte.
The researchers used a mechanical milling method to combine C and S to obtain composite particles (CS composite particles) that are highly dispersed in both nanometers. Since the complexed hydride LiBH4 is easily deformed, it is only necessary to pressurize the mixture of the CS composite particles and the LiBH4 particles to form a stable electrode/electrolyte interface capable of promoting the cell reaction at a high density, thereby producing a positive electrode having this interface. Layer (Figure 2).
This trial produced a solid-state ion battery using this CS composite particle as a positive electrode and metal Li as a negative electrode, and evaluated its characteristics. As a result, it was confirmed that the energy density per unit weight of the S positive electrode reached 1410 Wh·kg-1 or more, and the repeated charge and discharge of at least 45 times could be stably completed at a working temperature of 120° C. ( FIG. 3 ). (Reporter: Shinoda Yoshihiko)
Wire Mesh,Metal Wire Mesh,Ss Wire Mesh,Wire Mesh Netting
ANPING HONGYU WIREMESH CO.,LTD , https://www.hongyufence.com